Essure Birth Control Lawsuit & Recall
Last Updated: April 1, 2025
First marketed and sold in 2002, Essure, a permanently implanted birth control device, became a popular choice for female sterilization. Implanting the Essure device involved inserting a metal coil into each fallopian tube, inducing the creation of scar tissue and creating a blockage that stops eggs from traveling to the uterus This permanent contraception device could be inserted in as little as 15 minutes under local anesthesia, and was promoted as a less invasive alternative to procedures like tubal litigation.
Thousands of women began reporting serious Essure side effects and injuries, and dozens of deaths linked to Essure were reported to the FDA. Essure cases gained public attention through social media groups and a Netflix documentary, The Bleeding Edge. There was even a call for Essure market removal from celebrated lawyer Erin Brockovich. A massive number of Essure birth control lawsuits were filed against Bayer, the company that has manufactured Essure since 2013. In 2018, Essure was taken off the market.
Free Case Evaluation
If you have been harmed by using a Essure birth control device Select Justice can help you fight for your rights and compensation.
Bayer Essure Lawsuit
American medical products manufacturer and developer Conceptusmanufactured Essure from 2002-2013, before being bought for $1.1 billion by multinational pharmaceutical giant Bayer in 2013. As with Bayer’s purchase of Monsanto, which led to the Roundup weedkiller cancer lawsuits, Bayer soon learned that Conceptus came with countless problems.
In the following years, nearly 40,000 Essure lawsuits were filed against Bayer, leading to the company’s 2018 decision to pull the product off the market. While Bayer did not design Essure, the company was required to carry out due diligence on its products. Tens of thousands of women sustained Essure-related injuries, and there are reports of Essure-linked deaths. As the manufacturer of the product for five years, Bayer is accountable to women who trusted it to deliver safe and effective birth control.
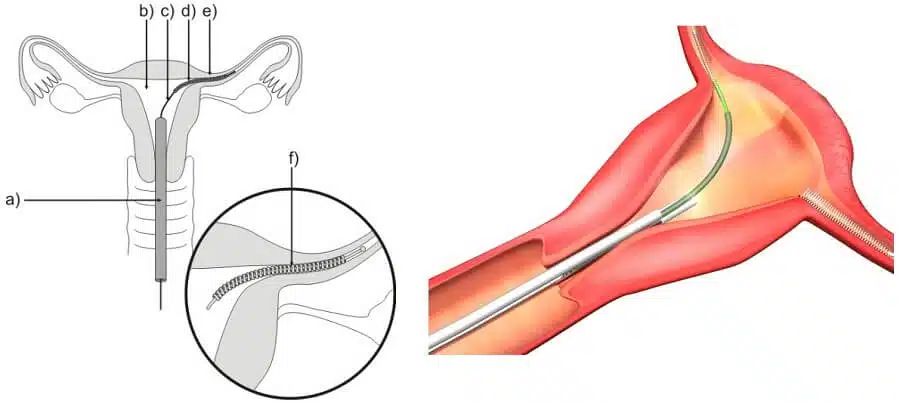
- After Essure, many women report experiencing pain and discomfort. This includes abdominal pain, back pain, and/or pelvic pain. Women have complained of pain that is severe enough to interfere with daily activities.
- Essure coils can migrate from their original location within the fallopian tube, perforating nearby organs such as the uterus and intestines. This can result in internal bleeding that requires surgical intervention.
- Essure is composed of nickel, a common allergen. Allergic reactions include hives, itching, and skin rashes.
- Essure is designed to be a permanent contraceptive, but there have been reports of unintended pregnancies after the procedure. These pregnancies may be ectopic, which means they occur outside of the uterus. A fetus cannot survive an ectopic pregnancy, and left untreated, an ectopic pregnancy can have life-threatening consequences for the mother.
- Some women reported heavier menstrual bleeding following their Essure procedure. For some women, heavy menstrual bleeding can lead to anemia.
- In some cases, women reported anxiety and depression following Essure implantation.
- Essure is permanent, unlike other contraceptives. Removing coils can be a complex and dangerous surgical procedure, and removal of the Essure device does not guarantee restored fertility.
To monitor the safety of medical devices, the FDA relies on reports about adverse events and product problems, FDA-mandated studies aftermarket, and published peer-reviewed literature. The FDA examined the information available about Essure, as well as the experiences of patients with Essure implants to determine whether the devices were safe enough for continued use. Due to an increasing number of reports regarding adverse reactions associated with Essure, the U.S. Food and Drug Administration required that Bayer add a black-box warning to the product's labeling. In 2018, Bayer finally announced it would stop selling Essure in the United States.
Essure Lawsuit News & Updates
April 1, 2025
To monitor the safety of medical devices, FDA relies on reports about adverse events and product problems, FDA-mandated studies after market, and published literature. The FDA examines the information available about Essure, as well as the experiences of patients with Essure implants. The FDA reviews the information about Essure and the experiences of patients with positive outcomes as well as those that experienced problems.
March 1, 2025
FDA Received 6,000 Reports About Essure in 2018
February 1, 2025
4 years ago Bayer agreed to a $1.6 billion settlement to resolve approximately 90% of the nearly 39,000 U.S. claims related to Essure. The settlement did not imply any admission of wrongdoing or liability by Bayer.
- January 1, 2025 - FDA Activities Related to Essure
- December 1, 2024 - Bayer to stop sales of Essure birth control device tied to injuries.
- November 1, 2024 - In 2024, Essure claims remain a significant area of concern for many individuals who experienced complications from the permanent birth control device. Essure, a non-surgical sterilization device, was designed to prevent pregnancy by inserting coils into the fallopian tubes, leading to scar tissue formation that blocks the tubes. However, many users reported severe side effects, such as chronic pain, heavy bleeding, autoimmune issues, and device migration, which have led to ongoing health problems. Though Bayer, the manufacturer, ceased sales of Essure in 2018, affected individuals have continued to seek compensation through lawsuits, claiming that the device's risks were inadequately disclosed. In 2024, legal efforts focus on ensuring adequate compensation for those who have suffered and raising awareness about long-term complications. This year, more support groups and legal resources have emerged to assist individuals in navigating the claims process, while healthcare professionals emphasize the need for informed decisions regarding contraceptive options.
- August 1, 2024 - The Essure contraceptive device continues to be the subject of litigation. Plaintiffs allege severe side effects, including pain, bleeding, and organ damage, which they claim were not adequately disclosed by the manufacturer. These cases are part of a broader wave of lawsuits that have been ongoing for several years.
- June 1, 2024 - Essure patients organized to report their problems to the FDA.
- May 1, 2024 - FDA puts restrictions on birth control implant but no recall.
- April 1, 2024 - Plasma and peritoneal fluid cytokine profiles in patient with Essure implant: Towards a molecular signature?
- March 1, 2024 - FDA Activities Related to Essure.
- February 1, 2024 - Female urogenital devices used during their lifetime-managing menstruation including pelvic health.
- January 1, 2024 - FDA received 6,000 reports about Essure in 2018 The FDA deemed the study on post-market surveillance of the controversial contraceptive device "adequate." The Essure arm has only 307 women enrolled.
- November 1, 2023 - Netflix's The Bleeding Edge Exposes Essure, Mesh and Other Dangerous Devices.
- July 19, 2023 - Clinical improvement after Essure devices removal: a systematic review.
- July 1, 2023 - Essure removal surgery: Is preoperative Transvaginal Ultrasonography and pelvic X ray required?
- May 1, 2023 - To monitor the safety of medical devices, FDA relies on reports about adverse events and product problems, FDA-mandated studies after market, and published literature. The FDA examines the information available about Essure, as well as the experiences of patients with Essure implants. The FDA reviews the information about Essure and the experiences of patients with positive outcomes as well as those who have experienced problems.
- December 1, 2022 - Bayer pharmaceutical agreed to a $2.5 billion settlement with American women over their horrific experiences with its contraceptive implant Essure device.
The most important Essure lawsuit update is arguably the settlement agreement in which Bayer agreed to pay $1.6 billion to claimants in August 2020. This was regarded as a resounding success for the Essure lawsuits, although Bayer did not admit any fault as part of the settlement.
What happens next?
Bayer’s Essure settlement covered around 90% of lawsuits filed at the time, but there is scope for more claimants to file lawsuits against Bayer for Essure. Indeed, the FDA updated its Essure advice webpage in October 2022, detailing the progress of Essure removal procedures in the United States. Another important note: Essure is distributed internationally, and women outside the U.S. are also pursuing Essure implant lawsuits.
Free Case Evaluation
If you have been harmed by using a Essure birth control device Select Justice can help you fight for your rights and compensation.
There currently isn’t an Essure FDA recall in place. Essure was removed from the market in the United States in 2018. After the reported problems with Essure, many women are electing to have the Essure implantation removed. There are numerous reasons to have an Essure removal procedure, including an attempt to reverse sterilization. However, many women have chosen to have the removal due to Essure’s side effects. There are also potential Essure removal side effects, leading to additional pain and suffering in women who have already faced Essure complications.
As stated, the FDA has never made an official Essure recall. However, the government watchdog has sounded several warnings over Essure. This includes the addition of a Black Box Warning on Essure packaging. A Black Box Warning – also known as a Black Label Warning – is an FDA initiative used to warn patients and healthcare professionals of possible serious complications, dangers, and adverse reactions to certain drugs and medical procedures. Please note, while there was no official Essure recall by the FDA, itdid provide guidance to healthcare providers that all unused Essure implant units should have been returned to Bayer by December 31, 2019.

Essure Complications & Side Effects
Essure injuries, complications, and side effects have been widely reported on multiple platforms. At the time of writing, the Essure Problems Facebook Group has over 43,000 members. Many similar groups have launched on social media in different regions of the world. The list of Essure problems is broad. Some will be particular to small numbers of women, while others are more widespread.
Some of the common Essure side effects and complications include:
Essure Side Effects List
- Pain/discomfort, particularly in the abdomen, pelvis, or back
- Fatigue
- Weight loss
- Bleeding
- Hair loss
- Depression
- Allergic reactions
- Perforation
The FDA lists both long- and short-term Essure side effects. Below, we have broken these down into two categories. Please note that both long-term and short-term side effects and complications can overlap.
Essure Side Effects After 10 Years
- Chronic pain
- Perforation
- Allergic reactions
- Unintended pregnancy (see below)
Essure Side Effects After 5 Years
- Cramping
- Bleeding
- Nausea
- Dizziness
- Headaches
- Pain & Discomfort
When side effects become severe, patients may require revision surgeries to mitigate the effects of the Essure device.
Nickel Poisoning Symptoms From Essure
It has been reported that Essure inserts can cause nickel poisoning, although the direct link between Essure and nickel poisoning has been contested in studies. Because the device is partly composed of nickel, some experts have warned about the nickel poisoning risk.
The Mayo Clinic lists the following symptoms of nickel poisoning:
- Rash or bumps on the skin
- Redness
- Itching
- Dry patches
- Blisters
Essure Contraceptive Complications
While no contraceptive solutions can be deemed 100% effective, Bayer touted the device’s 99.8% success rate in preventing pregnancy.. This claim has been contested by women opening lawsuits against Essure for unwanted pregnancies. The FDA points to clinical studies that show approximately 8% of women who undergo Essure implantation cannot rely on the device for birth control.
Bayer Essure Settlements & Payouts
There have been large-scale campaigns to secure compensation for women suffering from Essure implant complications. Essure justice became a celebrated campaign in the news media due to activists working on social media, as well as the release of documentaries on Netflix.
In 2020, Bayer agreed to pay $1.6 billion in compensation to settle around 90% of the Essure birth control lawsuits. As of late 2022, Bayer has yet to settle all the Essure cases, and the company is still facing legal action for injuries and deaths related to Essure in the United States and abroad.
If you believe you have been injured by Essure, you should contact an Essure injury lawyer to find out about your eligibility to secure compensation.
Essure Settlement Payout Amounts Per Person
In the first Bayer Essure payout of $1.6 billion, it was estimated that each claimant received somewhere in the region of $35,000-$40,000 per claim. This was based on the figure of around 40,000 claimants in the Bayer Essure lawsuit. While this does not mean future Essure lawsuits will secure that figure for new claimants, it serves as a useful reference point for estimating a possible Essure payout award.
If you have been injured by Essure, or are experiencing health complications following Essure implantation, speak to an Essure injury lawyer. As mentioned, Bayer has already agreed to pay around $1.6 billion in settlement money to claimants in previous Essure lawsuits. Essure injury law firms have already successfully secured compensation, so anyone with a claim should contact an attorney for expert advice on how to proceed.
How Do I Join the Essure Lawsuit
While Bayer has agreed to pay compensation to settle 90% of the Essure lawsuits brought by various law firms, it is still possible that those injured by Essure can pursue individual lawsuits. The best course of action is to speak to an experienced lawyer to see if you are eligible to make a claim.
Is Essure a Class Action Lawsuit or a Mass Tort Lawsuit?
Currently, the Essure lawsuits are not grouped together as a class action or mass tort suit. The settlement from Bayer in 2020 was used to pay a large group of claimants (around 40,000) to settle out of court. It is possible that more of the current Essure lawsuits become grouped together in a class action or mass tort case. Both types of litigation are used to group large numbers of cases together to streamline the process through the courts.
What Is Essure?
Essure is a metal coil implant used as birth control. The product was first launched by Concepto in 2002, which was bought by Bayer in 2013. After years of campaigning and lawsuits, Essure was removed from the market in 2018.
What is the Essure lawsuit about?
The Essure lawsuits were filed after thousands of women experienced problems, including injuries and side effects, linked to the birth control implant.
Is there a recall on Essure?
There is not currently an Essure recall. However, the FDA issued warnings about the product. Essure was removed from the market in the United States in 2018.
When Will Essure Settlements Be Paid?
In August 2020, Bayer set aside $1.6 billion to settle 90% of Essure lawsuits. If you are still waiting on your Essure payout award, you should contact your Essure law firm representative for advice.
Can You Get Pregnant With Essure?
No contraception method offers 100% results. Essure claimed to prevent 99.8% of pregnancies, although this was contested in the Essure lawsuits.
Why was Essure Taken Off the Market?
Bayer claimed Essure was taken off the market due to a lack of demand, something that was perhaps linked to the Essure complications awareness campaigns.
What problems does Essure cause?
Women who have used Essure have reported numerous problems and side effects. You can get the full list of Essure dangers on the FDA website.
Can you still sue Essure?
While Bayer settled 90% of Essure cases and no longer makes the product in the United States, it is still possible to pursue legal action against the company if you have been injured by Essure. For further advice, including your rights under the statute of limitations, contact a personal injury attorney.
Free Case Evaluation
If you have been harmed by using a Essure birth control device Select Justice can help you fight for your rights and compensation.

Medical Malpractice
Dangerous & Defective Products
Personal Injury Lawsuits
DUI Lawyer Support
Employer Negligence
Mass Tort Lawsuits
Top Class Action Lawsuits
Expertise
Reviews
FAQ







