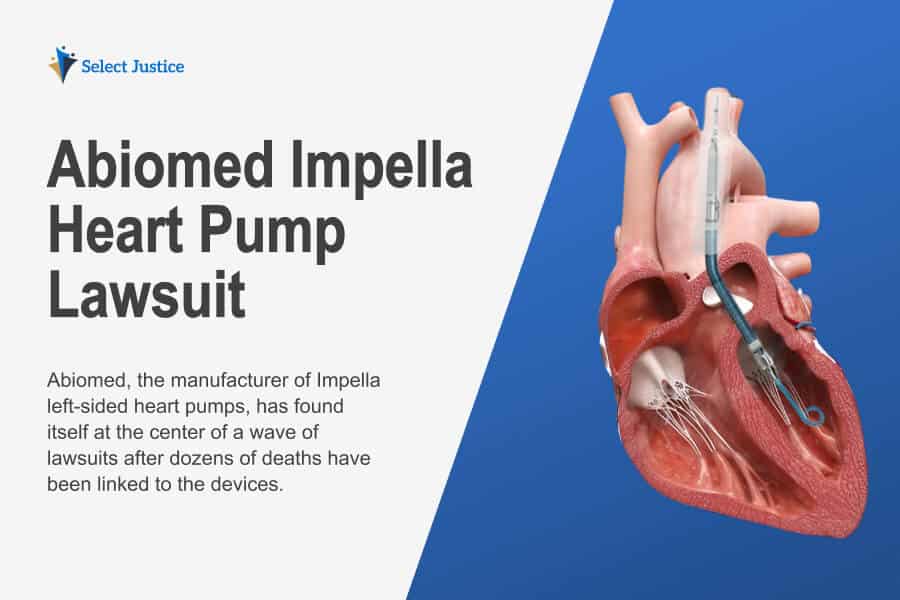
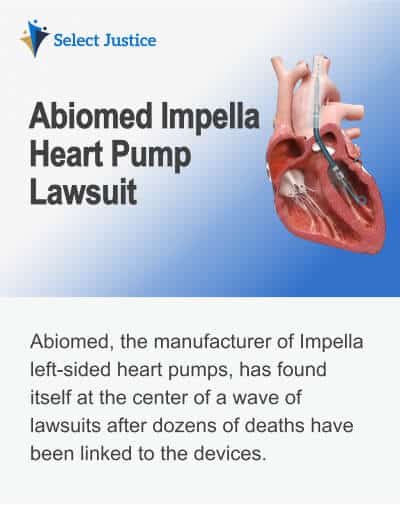
Abiomed, the manufacturer of Impella left-sided heart pumps, has found itself at the center of a wave of lawsuits after dozens of deaths have been linked to the devices. This issue concerns the instructions for the heart pumps, which do not adequately address the precautions required for insertion into the heart. At the time of writing, 49 deaths and 129 serious injuries have been reported by the FDA, which has issued a Class 1 Recall covering all Impella Heart Pumps. This is the most serious type of recall, one where the FDA cites a potential risk of death.
The lawsuits against Abiomed, which is an independent arm of Johnson & Johnson’s MedTech division, come from people who have undergone heart procedures such as TAVR (transcatheter aortic valve replacement). Impella heart pumps are intended to temporarily support the ventricles (the pumping chambers) during heart surgery procedures. The lawsuit claimants assert that the heart pumps have caused serious injuries, even death, as Abiomed failed to provide adequate instructions to surgeons for their correct insertion. The main risks come from perforation (tearing) of the walls of the heart, which can lead to serious injury and death. Patients who have had TAVR procedures may be at risk if the Impella motor comes into contact with a TAVR system stent and damage occurs to the motor.
Much of the recent focus on the Impella heart pump issues has centered on two areas: the FDA’s criticism of Abiomed for its actions after learning of the Impella heart pump risks and the fact that the devices remain on the market. While ostensibly the problem is with the instructions, not the devices, some in the media have called into question their continued use. The first lawsuits were filed against Abiomed in mid-2023, around the time of the first Impella heart pump recalls. A second device recall was initiated by Abiomed in December 2023. That timeline and Abiomed’s perceived inaction will be the focus of the lawsuits, particularly given that serious issues with Abiomed heart pumps have been reported since October 2021. The lawsuits are considered active, and we are awaiting trial dates. As of April 2024, lawyers are still accepting new cases for the Impella heart pump lawsuits.
As of April 2024, the FDA reports 49 deaths and 129 serious injuries linked to Impella heart pumps. It’s possible that more may follow. Around 66,000 Impella heart pumps are in use in the United States, with around 26,000 more worldwide. As mentioned, there is some criticism that this is a corrective recall, updating the 247-page instruction manual rather than recalling the device itself.
There have been two Impella heart pump recalls, both initiated by Abiomed and then later backed by the FDA. The first recall came in June 2023. It specifically dealt with TAVR patients, citing how the heart pump’s motor may impede the stent insert placed in the patient’s heart. Approximately 8,000 devices were recalled in June 2023. The second recall came on December 27th, 2023, and it was broader in scope in terms of how the device may endanger patients and on whom it may have an impact. Around 66,000 devices were recalled in December 2023.
Please note that both of these Impella heart pump recalls are considered product corrections, not product removals. However, this does not negate the severity of the recall (they are the FDA’s highest level of recall). Abiomed sent warning letters to thousands of patients detailing how the heart pumps should be corrected. If you have concerns, you should contact your healthcare provider immediately.
Manufacturing Dates for devices impacted on the first recall range from May 1st, 2021, to June 14th, 2023. The impacted devices listed by the FDA are:
- Impella 5.0 Blood Pump, Product Number 005062
- Impella CP Blood Pump, Product Number 0048-0032
- Impella 2.5 Blood Pump, Product Number 005042
- Impella CP with SmartAssist Blood Pump, Product Numbers 0048-0024, 0048-0045, 1000080
- Impella LD Blood Pump, Product Number 005082
- Impella 5.5 with SmartAssist Blood Pump, Product Numbers 0550-0008 And 1000100
Manufacturing Dates for devices impacted by the second recall range from October 10th, 2021, to October 10th, 2023. The impacted devices listed by the FDA* are:
- Impella 2.5
- Impella CP
- Impella CP with SmartAssist
- Impella 5.0
- Impella 5.5 with SmartAssist
- Impella LD
*specific product numbers are not listed on the FDA’s website
The Abiomed lawsuits are still at an early stage. There have been no settlements in the case as of yet, and lawyers are still asking for more potential claimants to come forward. Any payouts will depend on several factors, including the injuries caused by the Impella heart pumps, as well as any negligence on the part of Abiomed. Of course, it’s understandable that the families of those who have died or people who have been seriously injured should be entitled to substantial compensation. It’s worth noting that Abiomed has been in trouble over Impella heart pumps before, paying $3.1 million in 2018 after allegations of paying kickbacks to physicians for using the devices.
Lawyers are still accepting cases for the Abiomed heart pump lawsuits. If you or a loved one are among the thousands of Americans to have the Impella heart pump used in a procedure, you may be entitled to compensation. These lawsuits are at an early point in proceedings, and it is perfectly possible that new information will come to light, including more Impella heart pump recalls. If you or a family member had a heart procedure where the Abiomed Impella heart pump was used, speak to a lawyer to evaluate your case and see what your options are going forward.
What are the risks of Impella?
Nearly 50 deaths and dozens of serious injuries have been linked to Impella heart pumps. The FDA reports that the heart pumps may cause perforations in the heart, leading to serious injury and even death.
What is the main product of Abiomed?
Abiomed specializes in heart pumps and other support systems for patients with heart failure and related conditions.
What are the side effects of Impella heart pump?
In issuing a Class 1 Recall, the FDA is unequivocal that “Use of these devices may cause serious injuries or death”. The main issues are that the Impella heart pump may tear a hole in the chambers of the heart, or that it may interfere with other devices placed in the heart through surgery.
What is the Abiomed Impella heart pump lawsuit?
Claimants have filed lawsuits against Abiomed, maker of the Impella heart pump, after suffering injuries linked to the device. Abiomed admits that the instructions for the insertion of the devices were inadequate and issued two recalls. Lawyers say the mistakes made have cost lives. Injured parties and loved ones of those who died are taking legal action.
What are the vascular complications of Impella?
There can be several complications when inserting an Impella heart pump. A letter from Abiomed cautioned customers (physicians, surgeons, hospitals) on new operating procedures for the insertion of the device. The previous instructions were deemed inadequate, and they have been linked to many deaths and injuries.

Medical Malpractice
Dangerous & Defective Products
Personal Injury Lawsuits
DUI Lawyer Support
Employer Negligence
Mass Tort Lawsuits
Top Class Action Lawsuits
Expertise
Reviews
FAQ